3. Petroleum is a valuable resource because a small amount of it contains a large amount of energy. Petroleum also creates plastics and polymers.
5. Oil is crude because it is pumped from underground and cannot be used in its natural state without some degree of refinement. During refinement, it is separated into simpler mixtures through distillation.
6.
- a. 0.11 x 20,000,000= 2,200,000 barrels
- b. 0.89 x 20,000,000= 17,800,000 barrels
8. a. Water bottle, sports equipment, clothing, and artificial limbs.
b. A water bottle can be made out of aluminum, bamboo can be used to make light, flexible, and durable sports equipment, clothing can be made of cotton, and artificial limbs can be made of iron.
1o. a. The Middle East has the most petroleum reserves relative to its population.
b. Central Asia, Far East, and Oceania have the least petroleum reserves relative to its population.
11. a. North America, Central Asia, Far East, and Oceania, Western Europe, and Eastern Europe consume a greater proportion of the world’s supply of petroleum than they possess.
b. The Middle East, Africa, and Central and South America consume a smaller proportion of the world’s supply of petroleum than they possess.
12. If the substances are insoluble, density can be used to separate two different liquids.
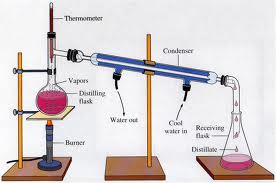
13. Water and acetone would be the easiest to separate from each other by distillation because water has the highest boiling point, and acetone has the lowest of the four substances, water and acetone would separate fairly quickly and easily by distillation.
15.
18. The highest boiling point in a distillation column would most likely be removed at the bottom because the thick liquids never vaporize.
20. Methane, pentane, hexane, octane. The higher the boiling point is, the stronger the intermolecular forces.
21. A covalent bond is the sharing of 2 or more valence electrons between 2 atoms, allowing both atoms to completely fill out their outer shells.
22. Atoms with filled electrons (8 valence electrons) are particularly stable, and therefore, tend to be chemically uncreative. Noble gases are atoms with filled outer electron shells.
23. Since the two dogs desire the sock, they must share it, although they desire to have it for themselves; like repelling electrons, the dogs pull away from each other, but are still connected by the bond they share with the sock connecting them.
a. A structural formula shows the makeup of a molecule, as well as how high the boiling point is, where as a molecular formula just shows the amount of atoms each element in the formula possesses.
b. The structure of a formula shows how strong molecular bonds within the formula is, as well as the boiling point of the formula.
27. Refer to drawings.
29.
- a. C9H20
- b. C16H34
- c. C10H22
- d. C18H38
30.
- a. 128g
- b. 226g
- c. 142g
- d. 254g
No comments:
Post a Comment