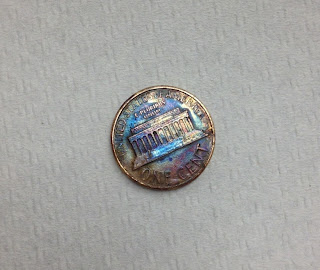Thursday, July 25, 2013
Feedback on the summer Chemistry course
1. The review sessions before the mini quizzes and tests have been extremely helpful, and worked really well this summer.
2. Some suggestions I have for tweaking or changing the source are, like we did today, to do the practice quiz before the mini quizzes. Also, another suggestion is for more review of the material that is going to be on the test.
3. I think that group presentations would be a much more interesting way of presenting the presentations. I like economist articles, but some other creative ideas like videos and performances, or songs, would be interesting. On the other hand, this might take more time. Personally, I liked the economist articles and I would leave that as it is.
4. We could use the blogs more effectively for studying for tests and finals. Everyone could find a group, and make a study guide with all the material. Then, everyone would use each others blogs and everyone would have most of the information.
5. Checking the homework would be good, but if it was not to be checked it would't make much of a difference. There is so much pressure of tests and quizzes, so if everyone had so much pressure on homework, everyone would be so worried and stressed and it isn't really worth it in the end.
6. More drawings, cool experiments without as many lab reports, and ideas like that would be a very creative approach to the new year. I think students would enjoy this very much.
2. Some suggestions I have for tweaking or changing the source are, like we did today, to do the practice quiz before the mini quizzes. Also, another suggestion is for more review of the material that is going to be on the test.
3. I think that group presentations would be a much more interesting way of presenting the presentations. I like economist articles, but some other creative ideas like videos and performances, or songs, would be interesting. On the other hand, this might take more time. Personally, I liked the economist articles and I would leave that as it is.
4. We could use the blogs more effectively for studying for tests and finals. Everyone could find a group, and make a study guide with all the material. Then, everyone would use each others blogs and everyone would have most of the information.
5. Checking the homework would be good, but if it was not to be checked it would't make much of a difference. There is so much pressure of tests and quizzes, so if everyone had so much pressure on homework, everyone would be so worried and stressed and it isn't really worth it in the end.
6. More drawings, cool experiments without as many lab reports, and ideas like that would be a very creative approach to the new year. I think students would enjoy this very much.
Tuesday, July 23, 2013
3SCS #1, 3, 6, 13 p.279, 3SBS #11 and 12, p.258
1. A. 1 repeating unit
B. 2 repeating units
C. 3 repeating units
D. 500-20,000 or more repeating units
3. Natural polymers: medicines, food additives, cotton, and silk
Synthetic polymers: celluloid, shellac, nylon, plastic toys
6. The term unsaturated is used to describe the structures of alkenes and alkynes because not all carbon atoms are bonded to their full capacity with four other atoms.
13.
––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––
11. A. ii
B. iii
C. i
D. ii
12. The other 75% is lost in heat, and this is why the engine is so inefficient.
B. 2 repeating units
C. 3 repeating units
D. 500-20,000 or more repeating units
3. Natural polymers: medicines, food additives, cotton, and silk
Synthetic polymers: celluloid, shellac, nylon, plastic toys
6. The term unsaturated is used to describe the structures of alkenes and alkynes because not all carbon atoms are bonded to their full capacity with four other atoms.
13.
––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––
11. A. ii
B. iii
C. i
D. ii
12. The other 75% is lost in heat, and this is why the engine is so inefficient.
3SBS #1-10 p.258
1. Petroleum is sometimes considered "buried sunshine" because as a fossil fuel, it originates from biomolecules of prehistoric plants and animals. The energy released by burning petroleum represents energy originally captured from sunlight by these prehistoric green plants during photosynthesis, hence "buried sunshine".
2. A) Potential energy is energy of position, or stored energy ready to be released. An example of potential energy is the energy within an unreleased winded up spring toy.
B) Kinetic energy is energy related to motion. A car rolling down a hill is an example of kinetic energy.
3. Chemical energy, another form of potential energy, is stored within the bonds in chemical compounds. When an energy-releasing reaction takes place, the bonds break and reactant atoms reorganize to form new bonds and release energy. If more energy is released than originally started with, the reaction is exothermic, but if less energy is released than originally started with, the reaction is endothermic.
4. A molecule of butane has more potential energy; it has more carbons than methane and a higher boiling point than methane. The bonds of butane are harder to break than the bonds of methane, resulting its higher potential energy.
5. A) Potential energy
B) Potential energy
C) Kinetic energy
D) Potential energy
E) Kinetic energy
6. Energy is required to break chemical bonds because it is what causes the reactant bonds to break and reorganize to form new bonds and energy.
7. A) Exothermic energy is released than is required to begin the chemical reaction.
B) Endothermic energy is required to crack large hydrocarbon molecules than is released.
C) Endothermic takes more energy to digest a candy bar than the energy released after digestion.
8. The product of a burning candle yields more energy than the energy to begin the reaction with an unlit candle. Since more energy is let off than required to begin the reaction, burning a candle is an exothermic reaction.
9.
10. The law of conservation of energy states that energy is neither created nor destroyed in any mechanical, physical, or chemical processes.
3SAS #31-38 p.235
31. A) Propane, C3H8
32. The suffix -ane indicates that hexane is an alkane.
33. Yes, each of these molecules are isomers of each other. They all consist of 5 carbon atoms and 12 hydrogen atoms. They appear to be different because of the arrangement of atoms. These three molecules are structured isomers of one another, due to the fact that they have identical molecular formulas, but different arrangements of atoms.
34.

35. Butane (C4H10) is the shortest-chain alkane that can demonstrate isomerism- alkanes with four or more carbon atoms can be demonstrated as straight-chain structures, branched-chain structures, and ring structures.
36. Both representations are correct because their different arrangements of atoms do not change the identical molecular formulas of the molecule; this would make the molecules structural isomers of each other.
37. A)
B) The branched-chain molecule would have the lower boiling point. Since the straight-chain molecule has greater molecule-to-molecule contact, it has a stronger intermolecular force than the branched-chain molecule, resulting in a higher boiling point.
38. A) A short, straight chain would have a lower boiling point because of decreased molecule-to-molecule contact than the longer boiling point. The bonds of this chain would be easier to break than a longer straight chain.
B) A short, branched chain would have the lower boiling point. Although the bonds of a branched chain are easier to break than those of a straight chain, it would be more difficult to break more molecular bonds within the long chain, resulting in a higher boiling point.
C) A short, branched chain would have a lower boiling point. Straight chains have stronger intermolecular forces that hold together each molecule in contact; where as bonds between branched chains are more breakable due to the decreased intermolecular molecular forces between them.
Monday, July 22, 2013
3SAS #1-30 p. 233 (except 2, 4, 9, 14, 24, 25)
1. A hydrocarbon- a molecular compound that contains carbon.
3. Petroleum is a valuable resource because a small amount of it contains a large amount of energy. Petroleum also creates plastics and polymers.
7. Heating and cooking fuel, petrochemicals, kerosene, refined oils, gas oil, heavy furnace oil, diesel fuel oil, lubricating oil and grease.
8. a. Water bottle, sports equipment, clothing, and artificial limbs.
b. A water bottle can be made out of aluminum, bamboo can be used to make light, flexible, and durable sports equipment, clothing can be made of cotton, and artificial limbs can be made of iron.
1o. a. The Middle East has the most petroleum reserves relative to its population.
b. Central Asia, Far East, and Oceania have the least petroleum reserves relative to its population.
11. a. North America, Central Asia, Far East, and Oceania, Western Europe, and Eastern Europe consume a greater proportion of the world’s supply of petroleum than they possess.
b. The Middle East, Africa, and Central and South America consume a smaller proportion of the world’s supply of petroleum than they possess.
12. If the substances are insoluble, density can be used to separate two different liquids.
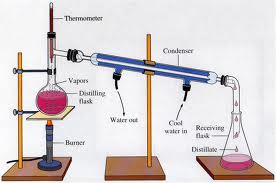
13. Water and acetone would be the easiest to separate from each other by distillation because water has the highest boiling point, and acetone has the lowest of the four substances, water and acetone would separate fairly quickly and easily by distillation.
15.
16. Fractional distillation differs from simple distillation because fractional distillation does not separate each substance in crude oil. Instead, it produces many different mixtures called fractions. Fractional distillation is a continuous process that consists of a distillation column and various temperatures in order to separate solutions. Simple distillation only involves a apparatus and separates a solution using evaporation and condensation.
17. Products derived from light include motor gasoline and refined oil. Products derived from intermediate include gas oil and heavy furnace oil. Products derived from heavy include lubricating oil/grease and heavy oils/wax.
18. The highest boiling point in a distillation column would most likely be removed at the bottom because the thick liquids never vaporize.
19. Distillation is a way to further separate the mixtures after fractional distillation.
20. Methane, pentane, hexane, octane. The higher the boiling point is, the stronger the intermolecular forces.
21. A covalent bond is the sharing of 2 or more valence electrons between 2 atoms, allowing both atoms to completely fill out their outer shells.
26.
a. A structural formula shows the makeup of a molecule, as well as how high the boiling point is, where as a molecular formula just shows the amount of atoms each element in the formula possesses.
b. The structure of a formula shows how strong molecular bonds within the formula is, as well as the boiling point of the formula.
27. Refer to drawings.
28.The electron-dot representation of a carbon atom only shows four dots because the four dots represent the valence electrons, located on the outer (and not inner) shell of the atom, where two electrons are located.
3. Petroleum is a valuable resource because a small amount of it contains a large amount of energy. Petroleum also creates plastics and polymers.
5. Oil is crude because it is pumped from underground and cannot be used in its natural state without some degree of refinement. During refinement, it is separated into simpler mixtures through distillation.
6.
- a. 0.11 x 20,000,000= 2,200,000 barrels
- b. 0.89 x 20,000,000= 17,800,000 barrels
8. a. Water bottle, sports equipment, clothing, and artificial limbs.
b. A water bottle can be made out of aluminum, bamboo can be used to make light, flexible, and durable sports equipment, clothing can be made of cotton, and artificial limbs can be made of iron.
1o. a. The Middle East has the most petroleum reserves relative to its population.
b. Central Asia, Far East, and Oceania have the least petroleum reserves relative to its population.
11. a. North America, Central Asia, Far East, and Oceania, Western Europe, and Eastern Europe consume a greater proportion of the world’s supply of petroleum than they possess.
b. The Middle East, Africa, and Central and South America consume a smaller proportion of the world’s supply of petroleum than they possess.
12. If the substances are insoluble, density can be used to separate two different liquids.
13. Water and acetone would be the easiest to separate from each other by distillation because water has the highest boiling point, and acetone has the lowest of the four substances, water and acetone would separate fairly quickly and easily by distillation.
15.
18. The highest boiling point in a distillation column would most likely be removed at the bottom because the thick liquids never vaporize.
20. Methane, pentane, hexane, octane. The higher the boiling point is, the stronger the intermolecular forces.
21. A covalent bond is the sharing of 2 or more valence electrons between 2 atoms, allowing both atoms to completely fill out their outer shells.
22. Atoms with filled electrons (8 valence electrons) are particularly stable, and therefore, tend to be chemically uncreative. Noble gases are atoms with filled outer electron shells.
23. Since the two dogs desire the sock, they must share it, although they desire to have it for themselves; like repelling electrons, the dogs pull away from each other, but are still connected by the bond they share with the sock connecting them.
a. A structural formula shows the makeup of a molecule, as well as how high the boiling point is, where as a molecular formula just shows the amount of atoms each element in the formula possesses.
b. The structure of a formula shows how strong molecular bonds within the formula is, as well as the boiling point of the formula.
27. Refer to drawings.
29.
- a. C9H20
- b. C16H34
- c. C10H22
- d. C18H38
30.
- a. 128g
- b. 226g
- c. 142g
- d. 254g
Unit 4 B.8 #1-6 p. 350
1. The mass of carbon dioxide produced daily by all 10 Riverwood High School buses is 1 kg.
2. 0.40 liters of fuel are consumed daily by buses idling at Riverwood High School.
3. In a 180-day school year, 180 kg of carbon dioxide would be released into the atmosphere by these buses while idling. On the other hand, 72 L of fuel would be consumed.
4. Atmospheric CO2 levels have increased by about 30% since 1800 due to clearing forests. Also, combustion of cuttings and scrap timber, and the burning of fossil fuels has to do with these atmospheric CO2 levels increasing.
5. No, because enough fossil fuel gas is produced naturally on its own.
6. The school bus-idling policy may seem necessary to some people in order to preserve the atmosphere. It also may seem necessary to prevent pollution.
2. 0.40 liters of fuel are consumed daily by buses idling at Riverwood High School.
3. In a 180-day school year, 180 kg of carbon dioxide would be released into the atmosphere by these buses while idling. On the other hand, 72 L of fuel would be consumed.
4. Atmospheric CO2 levels have increased by about 30% since 1800 due to clearing forests. Also, combustion of cuttings and scrap timber, and the burning of fossil fuels has to do with these atmospheric CO2 levels increasing.
5. No, because enough fossil fuel gas is produced naturally on its own.
6. The school bus-idling policy may seem necessary to some people in order to preserve the atmosphere. It also may seem necessary to prevent pollution.
Unit 4 B.3 #1-4 p. 344
1. Human exposure to ultraviolet radiation is more harmful than infrared radiation because infrared is simply heat energy, while ultraviolet, on the other hand, is a more energetic form of sun radiation. Ultraviolet radiation can cause sunburns and skin cancer. This harms many organisms through its ability to break covalent bonds.
2. Two essential roles played by visible solar radiation are that visible solar radiation energizes electrons in some chemical bonds. This supplies energy needed for photosynthesis reactions and the scattering of light in a sunset.
3. Due to less water vapor, there is less greenhouse gas caused by water vapor in dry and arid regions. Infrared radiation is not as stored and reradiated and reflected back into the atmosphere from the entering of UV and visible light that is transformed into IR radiation in clouds, but the visible and UV radiation directly exposed to the surface of the earth is used to heat earth.
4.
a. The daytime temperatures would be cooler, because with less greenhouse gases such as CO2 and H2O, less infrared radiation would be stored and reradiated and reflected back into the atmosphere from the transformation of UV and visible radiation to IR radiation in clouds. Less stored heat energy would enter throughout the day.
b. Nights would be very cold without the presence of the sun because there would be no stored heat energy in the atmosphere.
2. Two essential roles played by visible solar radiation are that visible solar radiation energizes electrons in some chemical bonds. This supplies energy needed for photosynthesis reactions and the scattering of light in a sunset.
3. Due to less water vapor, there is less greenhouse gas caused by water vapor in dry and arid regions. Infrared radiation is not as stored and reradiated and reflected back into the atmosphere from the entering of UV and visible light that is transformed into IR radiation in clouds, but the visible and UV radiation directly exposed to the surface of the earth is used to heat earth.
4.
a. The daytime temperatures would be cooler, because with less greenhouse gases such as CO2 and H2O, less infrared radiation would be stored and reradiated and reflected back into the atmosphere from the transformation of UV and visible radiation to IR radiation in clouds. Less stored heat energy would enter throughout the day.
b. Nights would be very cold without the presence of the sun because there would be no stored heat energy in the atmosphere.
Striking It Rich Lab Report
Striking It Rich Lab Report
Group Lithium: Nicolette, Nina, Makena
1. A. The untreated coin (the control) is copper in color. It is shiny. The coin that was heated in the zinc chloride solution turned silver on one side and had silver blotches on the other side. The coin that was heated on the hot plate after being in the zinc chloride solution turned gold on one side, and had purple blotches on the other side.
B. We know that there is Zinc because other forms of brass were shown.
2. The Law of Conservation of Matter proves that a coin composed of zinc and copper cannot react to form a precious metal, rather an alloy.
3. Zippers and jewelry would be two practical uses for these metallic changes because they can look like gold or silver just with this color change.
4. A. The copper atoms were still present; however, both zinc and copper combined to make an alloy.
B. Yes we think that the treated pennies could be converted back to ordinary coins using hydrochloric acid.
Sunday, July 21, 2013
Global Warming
Global warming is the term used to describe a gradual increase in the average temperature of the Earth's atmosphere and its oceans, a change that is believed to be permanently changing the Earth’s climate.
http://www.livescience.com/topics/global-warming/
http://www.livescience.com/topics/global-warming/
Unit 4 SBS #14-20 p.361
14. Since CO2 and water vapor are both greenhouse gases, they would absorb infrared radiation, helping maintain a stable temperature.
15. a. a. Natural process: Burning coal and removing trees in forests
Human activity: Breathing and photosynthesis
b. Natural process: Decomposition of plants and animals
Human process: Fossil fuels and livestock
15. a. a. Natural process: Burning coal and removing trees in forests
Human activity: Breathing and photosynthesis
b. Natural process: Decomposition of plants and animals
Human process: Fossil fuels and livestock
16. A) Lower- increase in greenhouse/atmospheric gases, higher air pressure.
B) Higher- decrease in greenhouse/atmospheric gases, lower air pressure.
17. On a sunny winter day, a greenhouse with transparent glass walls is warmer than a structure with dense wooden walls because infrared light cannot escape through the glass.
18. a.
19. Atmospheric CO2 gas, solid calcium carbonate, natural gas and organic molecules are all chemical reservoirs of carbon atoms.
b.
19. Atmospheric CO2 gas, solid calcium carbonate, natural gas and organic molecules are all chemical reservoirs of carbon atoms.
20. The carbon atom could be part of the atmosphere as a gas as a result of photosynthesis, in the lithosphere as plant or animal waste decay, or part of the hydrosphere as limestone.
Unit 4 BS #1-8 p.360
1. The frequency of electromagnetic radiation and its energy is proportional, but the wavelength is inversely proportional.
2. Spectrum is a good descriptor of the types of energy found in the electromagnetic radiation because it shows the ranges of energy.
3. Visible light is visible in plant photosynthesis while other forms of electromagnetic radiation are not because visible radiation can energize the electrons is chemical bonds, thus delivering the energy necessary for reactions.
4. a. Infrared, visible, and ultraviolet
b. Infrared radiation warms living things, visible reactions energize electrons, and ultraviolet radiation kills bacteria and destroys viruses.
5. Ultraviolet light is effective for this use because UV-C photons can break covalent bonds and leads to a chemical change in the materials exposed, and it also kills bacteria that damage other living things. On the other hand, visible light is not effective because it does not possess great enough energy.
6. -Infrared reactions can be absorbed by greenhouse gases, which reradiates back to earth. 90% of visible radiation travels to earth’s surface.
-UV-C radiation is absorbed in the stratosphere before reaching earth’s surface.
-Most UV-A and UV_B radiation is absorbed by the ozone layer.
7. Main effects of the solar radiation that reaches Earth's surface are that more greenhouse gases cause more infrared radiation, resulting in more energy reradiated on earths surface, and a hotter earth.
8. a. Asphalt warms up more when directly exposed to sunlight because when solar radiation reflects and illuminates it back into space. Lake water also reflects light, but it absorbs and stores heat.
b. Water acts as a greenhouse gas and absorbs infrared light, but asphalt directly reflects light sources back to space.
Thursday, July 18, 2013
What are the uses of alloys and how do their properties change?
Alloys are metals made by combining two or more metallic elements. Some of their uses include transportation, construction, ductility, aircraft turbine engines, and high corrosion resistance. Their properties change in ways such as a hardness of their steel forms, and the iron depends on the amount of the carbon added. Also, the change in iron depends on the processing it underwent.
2SDS 7-13 p.204
7. An alloy is a solid combination of atoms of two or more metals. Alloys also include some well-defined compounds.
8. Two alloys I use regularly are steel and 14-carat gold.
9. Carbon (C) is a component of both steel, and stainless steel.
10. Chromium-platinum alloy:
Formula: Cr3Pt
Use: basis of some commercial razor blade edges.
Physical property: very hard
8. Two alloys I use regularly are steel and 14-carat gold.
9. Carbon (C) is a component of both steel, and stainless steel.
10. Chromium-platinum alloy:
Formula: Cr3Pt
Use: basis of some commercial razor blade edges.
Physical property: very hard
11. Elements that behave as semiconductors are located on the break between metals and nonmetals--metalloids.
12. Three elements commonly used for doping semiconductors include phosphorus (P), arsenic (Ar), and aluminum (Al).
13. The primary use of the products of semiconductor technology is the allowance for computers to process digital information.
Wednesday, July 17, 2013
2SDS #1-6 p.204
1. Allotropes are forms of an element in the same state.
2. Oxygen and silicon also form allotropes.
3. a. A diamond is the hardest substance known, is is not electrically conductive, and it has an extremely high melting point. It is also rare, making it is very expensive. Coal is very combustible and cheap. Pencil lead, made of graphite, is a useful lubricant, a conductor of electricity, extremely soft, and very common and cheap.
b. Their properties are different because although they are made of the same element, they are allotropes of carbon, and therefore, have very different atomic arrangements. c. The rigid, three dimensional structure of carbon atoms in diamonds indicates its high melting point, hardness, and rareness that accounts for its high cost. The atomic makeup of graphite and coal indicate their much more common, more reactive, and softer properties, and therefore, their cheaper prices.
4. Engineered materials are materials developed by scientists and engineers to enhance natural materials through manufacturing methods that carefully control the microstructure of the materials. On the other hand, the makeup of natural materials, however, is uncontrolled and untouched.
5. Ceramics are durable and have high melting points and strength at high temperatures. However, ceramics are also brittle and when rapidly exposed to high and low temperatures, will crack or break.
6. Plastics can be customized to be either soft or hard. For example, polyethylene can be tailored to display soft properties, such as a squeeze bottle for water, or tailored to be hard and brittle, like glass. Plastic can also be made into optical fibers, which replace copper wires and provide noise free communication systems with high capacities.
2. Oxygen and silicon also form allotropes.
3. a. A diamond is the hardest substance known, is is not electrically conductive, and it has an extremely high melting point. It is also rare, making it is very expensive. Coal is very combustible and cheap. Pencil lead, made of graphite, is a useful lubricant, a conductor of electricity, extremely soft, and very common and cheap.
b. Their properties are different because although they are made of the same element, they are allotropes of carbon, and therefore, have very different atomic arrangements. c. The rigid, three dimensional structure of carbon atoms in diamonds indicates its high melting point, hardness, and rareness that accounts for its high cost. The atomic makeup of graphite and coal indicate their much more common, more reactive, and softer properties, and therefore, their cheaper prices.
4. Engineered materials are materials developed by scientists and engineers to enhance natural materials through manufacturing methods that carefully control the microstructure of the materials. On the other hand, the makeup of natural materials, however, is uncontrolled and untouched.
5. Ceramics are durable and have high melting points and strength at high temperatures. However, ceramics are also brittle and when rapidly exposed to high and low temperatures, will crack or break.
6. Plastics can be customized to be either soft or hard. For example, polyethylene can be tailored to display soft properties, such as a squeeze bottle for water, or tailored to be hard and brittle, like glass. Plastic can also be made into optical fibers, which replace copper wires and provide noise free communication systems with high capacities.
Tuesday, July 16, 2013
2SCS: p.181 #13-21
13. a. 6 moles NH3 are needed to react with 9 mol PbO.
b. 5 moles N2 are produced by the reaction of 10 mol NH3.
c. 5 moles Pb are produced from 5 mol PbO.
c. 28 g N2 can be produced from 34.0 g NH2.
d. 415 g PbO, which fully reacts, will produce 415 g Pb.
216+32=248. 216g/248g x 100%=
87% silver by mass
b.
molar masses: Al, 54g; O, 48g
54+48=102. 54g/102g x 100%=
53% aluminum by mass
c.
molar masses: Ca, 40g; C, 12g; O, 48g
40+12+48=100. 40g/100g x 100%=
40% calcium by mass
207+32+64=303. 207g/303g x 100%=
68% lead by mass
b.
5g/50g x 100%=
10% PbSO4 in the ore sample. c.
68 x .10=
6.8% Pb in the total ore sample. d.
b. 5 moles N2 are produced by the reaction of 10 mol NH3.
c. 5 moles Pb are produced from 5 mol PbO.
14. a. 1 mol N2 can be produced from 34.0 g NH3.
b. 621 g Pb can be produced from the complete reaction of 3.0 mol PbO.c. 28 g N2 can be produced from 34.0 g NH2.
d. 415 g PbO, which fully reacts, will produce 415 g Pb.
15. Due to the percent of the oxygen atoms being 67%, since oxygen's molar mass is 32 and carbon's molar mass is 12 in this molecule, the percent oxygen by mass 32g/44g x 100%, or 73%.
16. a.
molar masses: Ag, 216g; S, 32g216+32=248. 216g/248g x 100%=
87% silver by mass
b.
molar masses: Al, 54g; O, 48g
54+48=102. 54g/102g x 100%=
53% aluminum by mass
c.
molar masses: Ca, 40g; C, 12g; O, 48g
40+12+48=100. 40g/100g x 100%=
40% calcium by mass
17. a.
molar masses: Pb, 207g; S, 32g; O, 64g207+32+64=303. 207g/303g x 100%=
68% lead by mass
b.
5g/50g x 100%=
10% PbSO4 in the ore sample. c.
68 x .10=
6.8% Pb in the total ore sample. d.
18. a. Reusing is when you use the same product more than 1 time for the same thing, but recycling means the product is used again but for another reason or purpose.
b. Reusing- Clothes and Containers
Recycling- Old computer parts and Composed piles
19. a. Solar energy, biomass, wood, and natural gas
b. Coal, fossil fuels, food oil/petroleum, and gas
20. a. Reusing
b. Recycling
c. Recycling
21. A light bulb can only be recycled but a newspaper could be reused and recycled for different things.
Monday, July 15, 2013
2SCS p.180 #1-12
1. The law of conservation of matter states that matter is neither created nor destroyed.
2. A scientific law summarizes what has been learned by careful observation of nature.
3. These expressions are misleading because as the law of conservation of matter states, matter is neither created nor destroyed. They just change.
4. a. Not balanced.
Reactant: Sn-1, H-1, F-1 Product: Sn-1, H-2, F-2 b. Not balanced. Reactant: Si-1, O-2, C-1 Product: Si-1, O-1, C-2 c. Balanced Reactant: Al-1, O-3, H-6, Cl-3 Product: Al-1, O-3, H-6, Cl-3
5. a. The coefficient for hydrogen gas: 3. b. The coefficient for NH3 gas: 2. c. The coefficient for nitrogen gas: 1.
6. a. 1, 3, 1, 3
b. 2, 3, 3, 2
c. 4, 2, 3
7. a. 1 Ca3(PO4)2 + 3 H2SO4 --> 2 H3PO4 + 3 CaSO4 b. 2 C8H18 + 25 O2 --> 16 CO2 + 18 H2O
8. a. Yes, the atoms are conserved. Reactant: Na-2, S-1, O-4, K-2, Cl-1 Product: Na-2, S-1, O-4, K-2, Cl-1 b. No, the student did not create a properly balanced chemical equation. This is because the subscripts are suppose to stay the same, and the coefficients are suppose to alter to balance the equation. c. 1 Na2SO4 + 2 KCl --> 2 NaCl + 1 K2SO4
9. It would take 400,000 seconds to spend one mole of dollars if I could spend a billion dollars per second.
10. a. oxygen gas (O2): 32g b. ozone (O3): 48g c. limestone (CaCO3): 100g d. a typical antacid Mg(OH)2: 58g e. aspirin (C9H8O4): 180g
11. They can both correctly represent 1.00 mol of a substance because atomic mass equals atomic weight; the weight always stays the same.
12. a. 1 atom in 39.1g potassium b. .5 atoms in 19.55g potassium c. .1 atoms in 3.91g potassium d. .03 atoms in 1.0g potassium
2. A scientific law summarizes what has been learned by careful observation of nature.
3. These expressions are misleading because as the law of conservation of matter states, matter is neither created nor destroyed. They just change.
4. a. Not balanced.
Reactant: Sn-1, H-1, F-1 Product: Sn-1, H-2, F-2 b. Not balanced. Reactant: Si-1, O-2, C-1 Product: Si-1, O-1, C-2 c. Balanced Reactant: Al-1, O-3, H-6, Cl-3 Product: Al-1, O-3, H-6, Cl-3
5. a. The coefficient for hydrogen gas: 3. b. The coefficient for NH3 gas: 2. c. The coefficient for nitrogen gas: 1.
6. a. 1, 3, 1, 3
b. 2, 3, 3, 2
c. 4, 2, 3
7. a. 1 Ca3(PO4)2 + 3 H2SO4 --> 2 H3PO4 + 3 CaSO4 b. 2 C8H18 + 25 O2 --> 16 CO2 + 18 H2O
8. a. Yes, the atoms are conserved. Reactant: Na-2, S-1, O-4, K-2, Cl-1 Product: Na-2, S-1, O-4, K-2, Cl-1 b. No, the student did not create a properly balanced chemical equation. This is because the subscripts are suppose to stay the same, and the coefficients are suppose to alter to balance the equation. c. 1 Na2SO4 + 2 KCl --> 2 NaCl + 1 K2SO4
9. It would take 400,000 seconds to spend one mole of dollars if I could spend a billion dollars per second.
10. a. oxygen gas (O2): 32g b. ozone (O3): 48g c. limestone (CaCO3): 100g d. a typical antacid Mg(OH)2: 58g e. aspirin (C9H8O4): 180g
11. They can both correctly represent 1.00 mol of a substance because atomic mass equals atomic weight; the weight always stays the same.
12. a. 1 atom in 39.1g potassium b. .5 atoms in 19.55g potassium c. .1 atoms in 3.91g potassium d. .03 atoms in 1.0g potassium
Metal Report
Metal Report
Lithium
It's no myth, just call us lith.
Nicolette, Nina, and Makena
7.15.13
The production of lithium is a vital factor in the study of the metal. Lithium is found in the crystallized salt, and in the brine that underlies the crust. The recovery of lithium form hard rock metals through an open pit or underground hard rock mines using conventional mining techniques. The ore is then processed and concentrated using several methods before direct use or further processing into lithium compounds.
Some current uses for lithium include the following: mental illnesses, including bipolar disorder, depression, and schizophrenia; for eating disorders, including anorexia and bulimia; and for blood disorders, including anemia and low white-cell count (neutropenia). Although lithium is still used in bipolar disorder medications, it is less common nowadays.
Lithium is also used for headache, alcoholism, epilepsy, diabetes, liver disease,kidney disorders, arthritis, a skin condition called seborrhea, and overactive thyroid. Also, lithium is used in heat transfer applications and it is used as an alloying agent in synthesizing organic compounds.
Other uses include treatment of asthma, Huntington’s disease, Graves' disease,herpes simplex, a movement disorder called tardive dyskinesia, Tourette’s syndrome, cyclical vomiting, Meniere's disease, a tingling or “crawling” sensation in the skin (paresthesias), and aggressive behavior in people with attention deficit-hyperactivity disorder (ADHD).
Lithium is also used in batteries; however, due to its high cost, scientists are trying to replace this lithium ion battery into zinc air batteries which are less costly, more energy dense, and safer. Valporic acid mimics lithium, and is another alternative for treated bipolar disease.
Exactly how lithium works is unknown, but what we know is that it helps mental disorders by increasing the activity of chemical messengers in the brain.
Properties of
Lithium
|
Malleability and ductility: high
Electrical conductivity: high
Thermal conductivity: high
Chemical reactivity: high
Resistance to corrosion: low
Useful alloys formed: high
Color and luster: grey and metallic
|
Chemical Properties
of Lithium
|
Atomic number: 3
Atomic mass: 6.941
Density: 0.53 g.cm
Melting point: 180.5 °C
Boiling point: 1,342 °C
Isotopes: 2
Discovered by: Johann Arfvedson
|
There is a 92.5% natural abundance of lithium. Lithium is the 25th most abundant element on the earth. The uses and demands of lithium are met easily by the production of the metal, and meet the high demand.
Subscribe to:
Comments (Atom)