Nicolette's Chem Blog 2013
Thursday, July 25, 2013
Feedback on the summer Chemistry course
1. The review sessions before the mini quizzes and tests have been extremely helpful, and worked really well this summer.
2. Some suggestions I have for tweaking or changing the source are, like we did today, to do the practice quiz before the mini quizzes. Also, another suggestion is for more review of the material that is going to be on the test.
3. I think that group presentations would be a much more interesting way of presenting the presentations. I like economist articles, but some other creative ideas like videos and performances, or songs, would be interesting. On the other hand, this might take more time. Personally, I liked the economist articles and I would leave that as it is.
4. We could use the blogs more effectively for studying for tests and finals. Everyone could find a group, and make a study guide with all the material. Then, everyone would use each others blogs and everyone would have most of the information.
5. Checking the homework would be good, but if it was not to be checked it would't make much of a difference. There is so much pressure of tests and quizzes, so if everyone had so much pressure on homework, everyone would be so worried and stressed and it isn't really worth it in the end.
6. More drawings, cool experiments without as many lab reports, and ideas like that would be a very creative approach to the new year. I think students would enjoy this very much.
2. Some suggestions I have for tweaking or changing the source are, like we did today, to do the practice quiz before the mini quizzes. Also, another suggestion is for more review of the material that is going to be on the test.
3. I think that group presentations would be a much more interesting way of presenting the presentations. I like economist articles, but some other creative ideas like videos and performances, or songs, would be interesting. On the other hand, this might take more time. Personally, I liked the economist articles and I would leave that as it is.
4. We could use the blogs more effectively for studying for tests and finals. Everyone could find a group, and make a study guide with all the material. Then, everyone would use each others blogs and everyone would have most of the information.
5. Checking the homework would be good, but if it was not to be checked it would't make much of a difference. There is so much pressure of tests and quizzes, so if everyone had so much pressure on homework, everyone would be so worried and stressed and it isn't really worth it in the end.
6. More drawings, cool experiments without as many lab reports, and ideas like that would be a very creative approach to the new year. I think students would enjoy this very much.
Tuesday, July 23, 2013
3SCS #1, 3, 6, 13 p.279, 3SBS #11 and 12, p.258
1. A. 1 repeating unit
B. 2 repeating units
C. 3 repeating units
D. 500-20,000 or more repeating units
3. Natural polymers: medicines, food additives, cotton, and silk
Synthetic polymers: celluloid, shellac, nylon, plastic toys
6. The term unsaturated is used to describe the structures of alkenes and alkynes because not all carbon atoms are bonded to their full capacity with four other atoms.
13.
––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––
11. A. ii
B. iii
C. i
D. ii
12. The other 75% is lost in heat, and this is why the engine is so inefficient.
B. 2 repeating units
C. 3 repeating units
D. 500-20,000 or more repeating units
3. Natural polymers: medicines, food additives, cotton, and silk
Synthetic polymers: celluloid, shellac, nylon, plastic toys
6. The term unsaturated is used to describe the structures of alkenes and alkynes because not all carbon atoms are bonded to their full capacity with four other atoms.
13.
––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––
11. A. ii
B. iii
C. i
D. ii
12. The other 75% is lost in heat, and this is why the engine is so inefficient.
3SBS #1-10 p.258
1. Petroleum is sometimes considered "buried sunshine" because as a fossil fuel, it originates from biomolecules of prehistoric plants and animals. The energy released by burning petroleum represents energy originally captured from sunlight by these prehistoric green plants during photosynthesis, hence "buried sunshine".
2. A) Potential energy is energy of position, or stored energy ready to be released. An example of potential energy is the energy within an unreleased winded up spring toy.
B) Kinetic energy is energy related to motion. A car rolling down a hill is an example of kinetic energy.
3. Chemical energy, another form of potential energy, is stored within the bonds in chemical compounds. When an energy-releasing reaction takes place, the bonds break and reactant atoms reorganize to form new bonds and release energy. If more energy is released than originally started with, the reaction is exothermic, but if less energy is released than originally started with, the reaction is endothermic.
4. A molecule of butane has more potential energy; it has more carbons than methane and a higher boiling point than methane. The bonds of butane are harder to break than the bonds of methane, resulting its higher potential energy.
5. A) Potential energy
B) Potential energy
C) Kinetic energy
D) Potential energy
E) Kinetic energy
6. Energy is required to break chemical bonds because it is what causes the reactant bonds to break and reorganize to form new bonds and energy.
7. A) Exothermic energy is released than is required to begin the chemical reaction.
B) Endothermic energy is required to crack large hydrocarbon molecules than is released.
C) Endothermic takes more energy to digest a candy bar than the energy released after digestion.
8. The product of a burning candle yields more energy than the energy to begin the reaction with an unlit candle. Since more energy is let off than required to begin the reaction, burning a candle is an exothermic reaction.
9.
10. The law of conservation of energy states that energy is neither created nor destroyed in any mechanical, physical, or chemical processes.
3SAS #31-38 p.235
31. A) Propane, C3H8
32. The suffix -ane indicates that hexane is an alkane.
33. Yes, each of these molecules are isomers of each other. They all consist of 5 carbon atoms and 12 hydrogen atoms. They appear to be different because of the arrangement of atoms. These three molecules are structured isomers of one another, due to the fact that they have identical molecular formulas, but different arrangements of atoms.
34.

35. Butane (C4H10) is the shortest-chain alkane that can demonstrate isomerism- alkanes with four or more carbon atoms can be demonstrated as straight-chain structures, branched-chain structures, and ring structures.
36. Both representations are correct because their different arrangements of atoms do not change the identical molecular formulas of the molecule; this would make the molecules structural isomers of each other.
37. A)
B) The branched-chain molecule would have the lower boiling point. Since the straight-chain molecule has greater molecule-to-molecule contact, it has a stronger intermolecular force than the branched-chain molecule, resulting in a higher boiling point.
38. A) A short, straight chain would have a lower boiling point because of decreased molecule-to-molecule contact than the longer boiling point. The bonds of this chain would be easier to break than a longer straight chain.
B) A short, branched chain would have the lower boiling point. Although the bonds of a branched chain are easier to break than those of a straight chain, it would be more difficult to break more molecular bonds within the long chain, resulting in a higher boiling point.
C) A short, branched chain would have a lower boiling point. Straight chains have stronger intermolecular forces that hold together each molecule in contact; where as bonds between branched chains are more breakable due to the decreased intermolecular molecular forces between them.
Monday, July 22, 2013
3SAS #1-30 p. 233 (except 2, 4, 9, 14, 24, 25)
1. A hydrocarbon- a molecular compound that contains carbon.
3. Petroleum is a valuable resource because a small amount of it contains a large amount of energy. Petroleum also creates plastics and polymers.
7. Heating and cooking fuel, petrochemicals, kerosene, refined oils, gas oil, heavy furnace oil, diesel fuel oil, lubricating oil and grease.
8. a. Water bottle, sports equipment, clothing, and artificial limbs.
b. A water bottle can be made out of aluminum, bamboo can be used to make light, flexible, and durable sports equipment, clothing can be made of cotton, and artificial limbs can be made of iron.
1o. a. The Middle East has the most petroleum reserves relative to its population.
b. Central Asia, Far East, and Oceania have the least petroleum reserves relative to its population.
11. a. North America, Central Asia, Far East, and Oceania, Western Europe, and Eastern Europe consume a greater proportion of the world’s supply of petroleum than they possess.
b. The Middle East, Africa, and Central and South America consume a smaller proportion of the world’s supply of petroleum than they possess.
12. If the substances are insoluble, density can be used to separate two different liquids.
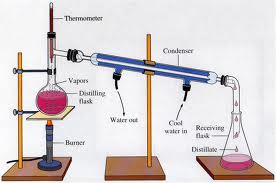
13. Water and acetone would be the easiest to separate from each other by distillation because water has the highest boiling point, and acetone has the lowest of the four substances, water and acetone would separate fairly quickly and easily by distillation.
15.
16. Fractional distillation differs from simple distillation because fractional distillation does not separate each substance in crude oil. Instead, it produces many different mixtures called fractions. Fractional distillation is a continuous process that consists of a distillation column and various temperatures in order to separate solutions. Simple distillation only involves a apparatus and separates a solution using evaporation and condensation.
17. Products derived from light include motor gasoline and refined oil. Products derived from intermediate include gas oil and heavy furnace oil. Products derived from heavy include lubricating oil/grease and heavy oils/wax.
18. The highest boiling point in a distillation column would most likely be removed at the bottom because the thick liquids never vaporize.
19. Distillation is a way to further separate the mixtures after fractional distillation.
20. Methane, pentane, hexane, octane. The higher the boiling point is, the stronger the intermolecular forces.
21. A covalent bond is the sharing of 2 or more valence electrons between 2 atoms, allowing both atoms to completely fill out their outer shells.
26.
a. A structural formula shows the makeup of a molecule, as well as how high the boiling point is, where as a molecular formula just shows the amount of atoms each element in the formula possesses.
b. The structure of a formula shows how strong molecular bonds within the formula is, as well as the boiling point of the formula.
27. Refer to drawings.
28.The electron-dot representation of a carbon atom only shows four dots because the four dots represent the valence electrons, located on the outer (and not inner) shell of the atom, where two electrons are located.
3. Petroleum is a valuable resource because a small amount of it contains a large amount of energy. Petroleum also creates plastics and polymers.
5. Oil is crude because it is pumped from underground and cannot be used in its natural state without some degree of refinement. During refinement, it is separated into simpler mixtures through distillation.
6.
- a. 0.11 x 20,000,000= 2,200,000 barrels
- b. 0.89 x 20,000,000= 17,800,000 barrels
8. a. Water bottle, sports equipment, clothing, and artificial limbs.
b. A water bottle can be made out of aluminum, bamboo can be used to make light, flexible, and durable sports equipment, clothing can be made of cotton, and artificial limbs can be made of iron.
1o. a. The Middle East has the most petroleum reserves relative to its population.
b. Central Asia, Far East, and Oceania have the least petroleum reserves relative to its population.
11. a. North America, Central Asia, Far East, and Oceania, Western Europe, and Eastern Europe consume a greater proportion of the world’s supply of petroleum than they possess.
b. The Middle East, Africa, and Central and South America consume a smaller proportion of the world’s supply of petroleum than they possess.
12. If the substances are insoluble, density can be used to separate two different liquids.
13. Water and acetone would be the easiest to separate from each other by distillation because water has the highest boiling point, and acetone has the lowest of the four substances, water and acetone would separate fairly quickly and easily by distillation.
15.
18. The highest boiling point in a distillation column would most likely be removed at the bottom because the thick liquids never vaporize.
20. Methane, pentane, hexane, octane. The higher the boiling point is, the stronger the intermolecular forces.
21. A covalent bond is the sharing of 2 or more valence electrons between 2 atoms, allowing both atoms to completely fill out their outer shells.
22. Atoms with filled electrons (8 valence electrons) are particularly stable, and therefore, tend to be chemically uncreative. Noble gases are atoms with filled outer electron shells.
23. Since the two dogs desire the sock, they must share it, although they desire to have it for themselves; like repelling electrons, the dogs pull away from each other, but are still connected by the bond they share with the sock connecting them.
a. A structural formula shows the makeup of a molecule, as well as how high the boiling point is, where as a molecular formula just shows the amount of atoms each element in the formula possesses.
b. The structure of a formula shows how strong molecular bonds within the formula is, as well as the boiling point of the formula.
27. Refer to drawings.
29.
- a. C9H20
- b. C16H34
- c. C10H22
- d. C18H38
30.
- a. 128g
- b. 226g
- c. 142g
- d. 254g
Subscribe to:
Comments (Atom)