Striking It Rich Lab Report
Group Lithium: Nicolette, Nina, Makena
1. A. The untreated coin (the control) is copper in color. It is shiny. The coin that was heated in the zinc chloride solution turned silver on one side and had silver blotches on the other side. The coin that was heated on the hot plate after being in the zinc chloride solution turned gold on one side, and had purple blotches on the other side.
B. We know that there is Zinc because other forms of brass were shown.
2. The Law of Conservation of Matter proves that a coin composed of zinc and copper cannot react to form a precious metal, rather an alloy.
3. Zippers and jewelry would be two practical uses for these metallic changes because they can look like gold or silver just with this color change.
4. A. The copper atoms were still present; however, both zinc and copper combined to make an alloy.
B. Yes we think that the treated pennies could be converted back to ordinary coins using hydrochloric acid.




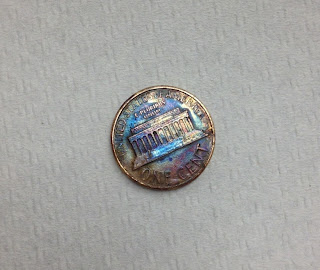

No comments:
Post a Comment